By Rosalind Brown, Centre for Clinical Brain Sciences, University of Edinburgh
Most people will have heard of Alzheimer’s disease, but fewer people are aware of cerebral small vessel disease (SVD), a silently progressing disease of ageing responsible for nearly half of all cases of dementia along with a large percentage of strokes. The global health impact of SVD is huge and while a small number of genetic mutations have been identified as causing some forms, for the majority of cases the cause is uncertain.
Importantly, there is currently no cure.
But it’s not all doom and gloom! SVD is a challenging, complex disorder, but researchers have made significant developments in recent years and are working towards building a clearer understanding of SVD and identifying new treatment targets.
What are the outstanding questions?
There are a lot of things about SVD that we still need to understand, from differences in disease manifestations between patients to the cellular and molecular changes that underlie disease progression. A few of the broader questions are:
What causes SVD? To treat SVD, we need to understand what causes it. We still don’t know what causes most cases, and why some people with neuroimaging signs of SVD develop symptoms and others don’t. But progress is being made – for example, we know that genetic mutations underlie several forms of familial SVD, but recent studies have shown that genetic factors also contribute to so-called sporadic SVD.
Which cells and tissues are involved? While the endothelium is known to be a major player in SVD, new research points to roles for oligodendrocytes, the basement membrane and extracellular matrix. Determining the cell types and processes involved in the pathogenesis of SVD will be important for developing treatments.
Are we using appropriate preclinical models? Several rodent models are used to study SVD including the spontaneously hypertensive stroke prone rat (SHRSP) and CADASIL mouse model. While we don’t have a model that captures all aspects of SVD, they do reproduce some separate features of the disease and, if used appropriately, will continue to improve our understanding. However, the limitations of each model need to be carefully considered and we need to be careful to use suitable models to ensure valid translation into a clinical setting.
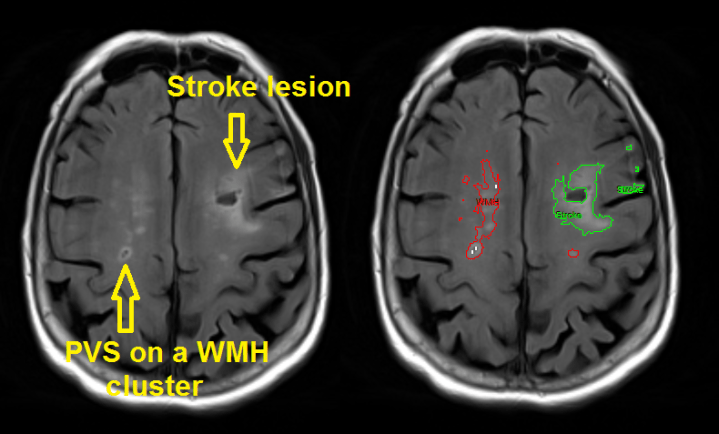
What else can we learn from clinical studies? Clinical brain imaging studies have been leading the way in the field of SVD, and several new and exciting avenues for research are becoming apparent. For example, brain lesion location is surprisingly overlooked but has now been identified as a potential important indicator for the outcome of SVD. The potential ability to predict future outcome (e.g. dementia) from lesion characteristics is an exciting prospect, particularly for early therapeutic treatments, and one that merits further investigation.

Pathophysiology in Binswanger’s disease (BD), a form of SVD – Clinical Science, Rosenberg GA 2017
(A) FLAIR MRI showing extensive damage to the white matter (WM). (B) Myelin-stained patient brain, with long-standing poorly treated hypertension, showing demyelination in the centrum semiovale (indicated by*), which spares the association U-fibres (arrow). (C) BBB permeability study in a BD patient using dynamic contrast-enhanced MRI. The red areas are increased permeability, the white areas are WM hyperintensities (WMHs), the green areas are a rim of 4 mm around the WMHs. Most of the increased permeabillty is found around the edge of the WMHs and in the normal appearing WM. (D) Enlargement of the WMH region in C showing the increased permeability around the injury area.
If scientists can address these questions, we may improve our understanding of how to treat SVD. A recent special edition of Clinical Science on small vessels, dementia and chronic disease presents a collection of articles that tackle these outstanding questions (and more) and provides significant food for thought in this developing field.
Collaboration will be key!
We’ve made progress, but there is a lot that we don’t understand! SVD is beginning to move into the limelight and the impact it has on global health is more widely acknowledged. It is clear that there are many avenues for investigation and getting to the bottom of this disorder will require communication between international experts in the field from multiple disciplines.
Further reading:
Small vessels, dementia & chronic disease themed collection – Clinical Science
Perivascular space in cerebral small vessel disease international network website
The University of Edinburgh is a leading institution for research in small vessel disease, dementia and stroke. Rosalind Brown is Scientific Research Coordinator at the Centre for Clinical Brain Sciences in the College of Medicine, highly integrated in the local Edinburgh Neuroscience community.

